An Interest In:
Web News this Week
- April 23, 2024
- April 22, 2024
- April 21, 2024
- April 20, 2024
- April 19, 2024
- April 18, 2024
- April 17, 2024
August 23, 2020 06:42 pm
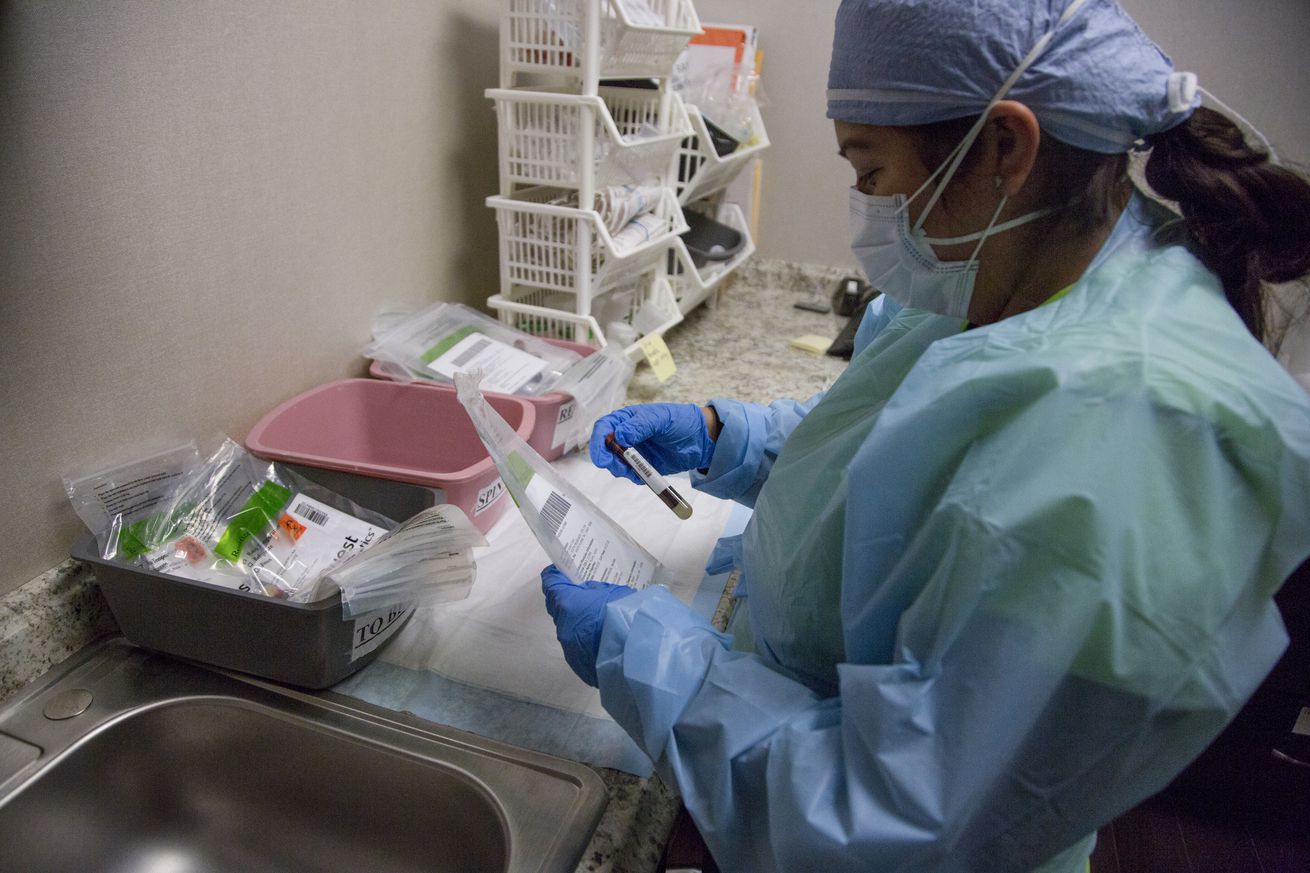
A medical worker in New York checks blood for coronavirus antibodies. | Photo by Pablo Monsalve / VIEWpress via Getty Images
Original Link: https://www.theverge.com/2020/8/23/21347251/covid-19-plasma-treatment-antibodies-fda-authorization-trump
FDA authorizes convalescent plasma to treat COVID-19

A medical worker in New York checks blood for coronavirus antibodies. | Photo by Pablo Monsalve / VIEWpress via Getty Images
The Food and Drug Administration authorized the emergency use of blood plasma from COVID-19 survivors as a treatment for sick patients. The announcement came after President Donald Trump publicly pressured the agency to speed the development of drugs and vaccines.
Plasma should not be the new standard of care for COVID-19, the agency said in a statement. However, it may shorten or reduce the severity of the illness in hospitalized patients. “We’re encouraged by the early promising data that we’ve seen about convalescent plasma,” said commissioner Stephen Hahn in the statement.
Preliminary research suggests that the plasma, which contains antibodies against the coronavirus, could help improve the survival rate of people hospitalized...
Original Link: https://www.theverge.com/2020/8/23/21347251/covid-19-plasma-treatment-antibodies-fda-authorization-trump
Share this article:
Tweet

View Full Article
The Verge
 The Verge is an ambitious multimedia effort founded in 2011
The Verge is an ambitious multimedia effort founded in 2011More About this Source Visit The Verge

